Infertility in women
Highlights
Infertility in Women
In the U.S., about 10% of women ages 15 - 44, or about 6.1 million women, have difficulty getting pregnant or carrying a baby to term.
Risk Factors
Risk factors for female infertility include:
- Age. Fertility begins to decline when a woman reaches her mid-30s, and rapidly declines after her late 30s.
- Weight. Extreme weight levels, either high or low, can contribute to infertility.
- Smoking. Cigarette smoking can impair a woman’s fertility.
Causes
Infertility may be caused by an underlying medical condition that damages the fallopian tubes, interferes with ovulation, or causes hormonal complications. These medical conditions include:
- Pelvic inflammatory disease
- Endometriosis
- Polycystic ovary syndrome
- Premature ovarian failure
- Uterine fibroids
Diagnosis
If you have been unable to conceive after 1 year of unprotected sex, talk with your doctor about having your fertility evaluated. Fertility testing should especially be performed if a woman is over 35 years old or if either partner has known risk factors for infertility. An analysis of the man's semen should be performed before the female partner undergoes any invasive testing.
Treatment
Treatment for infertility should first address any underlying medical condition that may be contributing to fertility problems. If this step does not restore fertility, there are several treatment approaches:
- Lifestyle measures (such as maintaining a healthy weight, quitting smoking, timing sexual activity with regard to the ovulation cycle)
- Drugs to induce ovulation, such as clomiphene and gonadotrophins
- Assisted reproductive technologies (ART), such as in vitro fertilization (IVF)
Introduction
Infertility is the failure of a couple to become pregnant after one year of regular, unprotected intercourse. In both men and women the fertility process is complex.
Infertility affects about 10% of all couples. About a third of infertility problems are due to female infertility, and another third are due to male infertility. In the remaining cases, infertility affects both partners or the cause is unclear. Although this report specifically addresses infertility in women, it is important for the male partner to be tested at the same time. [For more information, see In-Depth Report #67: Infertility in men.]
The Female Reproductive System
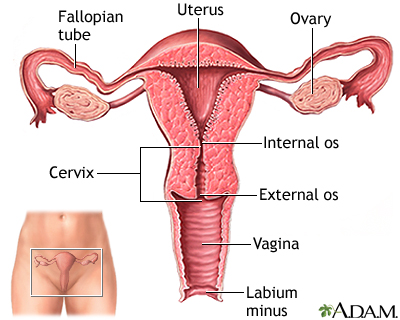
The primary organs and structures in the reproductive system are:
- The uterus is a pear-shaped organ located between the bladder and lower intestine. It consists of two parts, the body and the cervix.
- The cervix is the lower portion of the uterus. It has a canal opening into the vagina with an opening called the os, which allows menstrual blood to flow out of the uterus into the vagina.
- Leading off each side of the uterus are two tubes known as the fallopian tubes. Near the end of each tube is an almond-sized organ called an ovary. Women have two ovaries, one located on each side of the uterus.
- Ovaries are the ”factories” for egg production. Ovaries contain hundreds of thousands of follicles (from folliculus, meaning "sack" in Latin). Each ovarian follicle houses an immature egg. .
- At the beginning of a menstrual cycle, several ovarian follicles begin to develop, but only one follicle becomes dominant. The dominant follicle produces a mature egg, which is released at the time of ovulation. Usually, only one egg is released. (Multiple births occur if two or more eggs are released and fertilized. Fraternal twins develop from two separate eggs that are fertilized by two separate sperm. Identical twins develop from a single fertilized egg that splits to form two embryos.)
Reproductive Hormones
The menstrual cycle is regulated by the complex surge and fluctuations of many different reproductive hormones, which work together to prepare a women’s body for pregnancy.
The hypothalamus (an area in the brain) and the pituitary gland control six important hormones:
- Gonadotropin-releasing hormone (GnRH) is released by the hypothalamus.
- GnRH stimulates the pituitary gland to produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
- Estrogen, progesterone, and the male hormone testosterone are secreted by the ovaries at the command of FSH and LH.
The Menstrual Cycle
During a woman’s monthly menstrual cycle, her body prepares for conception and pregnancy. The average menstrual cycle is about 28 days but anywhere from 21 days to 35 days is considered normal. The menstrual cycle is divided into three phases: Follicular, Ovulatory, and Luteal.
Follicular Phase. The follicular phase begins with the first day of menstrual bleeding:
- At the start of the follicular phase, estrogen and progesterone levels are at their lowest point. This causes the uterine lining to break down and shed.
- At the same time, the hypothalamus produces GnRH, which stimulates the production of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH trigger the production of estrogen.
- As FSH levels increase, they stimulate the growth and maturation of eggs in the follicles. About 15 - 20 follicles are stimulated, but only one follicle continues to mature.
- The dominant follicle produces estrogen. The other follicles stop growing and disintegrate.
Ovulatory Phase. The ovulatory phase occurs halfway through the menstrual cycle (about 14 days after the start of the follicular phase.) Ovulation, the critical process for conception, occurs during the ovulatory phase. A woman’s fertile period starts about 3 - 5 days before ovulation and ends 24 - 48 hours after it. During the ovulatory phase:
- The increase in estrogen from the dominant follicle triggers a surge in LH. As estrogen levels rise, they also prompt the cervix to secrete more mucus to help nourish and propel sperm to the egg.
- The LH surge signals the dominant follicle to burst and release the developed egg into the fallopian tube. The release of the egg is called ovulation. Once in the fallopian tube, the egg is in place for fertilization.
- The egg can live for 24 - 48 hours after being released. (Sperm can live for 3 - 5 days.) A woman is most likely to get pregnant if sex occurs in the 3 - 5 days before ovulation or on the day of ovulation.
Luteal Phase. The luteal phase begins immediately after ovulation and ends when the next menstrual period starts. The luteal phase lasts about 12 - 16 days. During the luteal phase:
- After releasing the egg, the ruptured follicle closes and forms corpus luteum, a yellow mass of cells that provide a source of estrogen and progesterone during pregnancy. These hormones help the uterine lining to thicken and prepare for the egg’s implantation.
- If the egg is fertilized by a sperm cell, it implants in the uterus and pregnancy begins.
- If fertilization does not occur, the egg breaks apart. The corpus luteum degenerates, and estrogen and progesterone levels drop.
- Finally, the thickened uterine lining sloughs off and is shed along with the unfertilized egg during menstruation. The menstrual cycle begins again.
Fertilization and Pregnancy
Conception occurs when an egg is fertilized by a sperm. The so-called "fertile window" is about 6 days long. It starts about 5 days before ovulation and ends the day of ovulation. Fertilization occurs as follows:
- Sperm can survive for 3 - 5 days afterthey enter the fallopian tube. The egg survives –24 - 48 hours unless it is fertilized by a sperm.
- The fertilized egg is called a zygote. The zygote immediately begins to divide until it becomes a ball of cells known as a blastocyst.
- The blastocyst moves from the fallopian tube into the uterus where it is implanted in the uterine lining. Implantation takes place about 6 - 10 days after fertilization. Implantation is when pregnancy begins.
- The inner cells of the blastocyst becomes the embryo, which develops into the fetus. The outer cells of the blastocyst becomes the placenta. The placenta is a thick blanket of blood vessels that nourishes the fetus as it develops.
- The developing embryo produces and secretes the protein human chorionic gonadotropin (hCG), which helps signal the corpus luteum (the yellow tissue formed from the ruptured follicle) to continue to produce estrogen and progesterone. HCG is the hormone detected by pregnancy tests. After about 10 weeks, the placenta takes over production of progesterone and estrogen, and the corpus luteum degenerates.
Typical Menstrual Cycle | ||
Menstrual Phases | Typical No. of Days | Hormonal Actions |
Follicular (Proliferative) Phase | Cycle Days 1 - 6: Beginning of menstruation to end of blood flow. | Estrogen and progesterone start out at their lowest levels. FSH levels rise to stimulate maturity of follicles. Ovaries start producing estrogen and levels rise, while progesterone levels remains low. |
Cycle Days 7 - 13: The endometrium (the inner lining of the uterus) thickens to prepare for egg implantation. | ||
Ovulation | Cycle Day 14: | Surge in LH. Largest follicle bursts and releases egg into fallopian tube. |
Luteal (Secretory) Phase, also known as the Premenstrual Phase | Cycle Days 15 - 28: | Ruptured follicle develops into corpus luteum, which produces progesterone. Progesterone and estrogen stimulate blanket of blood vessels to prepare for egg implantation. |
If fertilization occurs: | Fertilized egg attaches to blanket of blood vessels that supplies nutrients for the developing placenta. Corpus luteum continues to produce estrogen and progesterone. | |
If fertilization does not occur: | Corpus luteum deteriorates. Estrogen and progesterone levels drop. The blood vessel lining sloughs off and menstruation begins. | |
Causes
Most cases of female infertility are due to medical conditions that cause:
- Ovulation problems
- Blocked fallopian tubes
- Structural problems in the reproductive system
- Problems with quality of cervical mucus or eggs
Ovulation Problems
Ovulation is the release of the egg that occurs during the monthly menstrual cycle. Problems that affect ovulation, and the hormones involved with ovulation, are the most common cause of female infertility. They include:
- Polycystic Ovarian Syndrome (PCOS). Women with PCOS do not ovulate regularly and they experience infrequent or absent menstrual cycles. In PCOS, the ovaries produce excessive amounts of androgens (male hormones), particularly testosterone. Increased androgen production results in high levels of luteinizing hormone (LH) and low levels of follicle-stimulating hormone (FSH), so that follicles are prevented from producing a mature egg. Without egg production, the follicles swell with fluid and form into cysts. The elevated levels of androgens can also cause obesity, facial hair, and acne. PCOS poses a high risk for insulin resistance, which is associated with type 2 diabetes.
- Premature Ovarian Failure (Early Menopause). Premature ovarian failure is the early depletion of follicles before age 40, which, in most cases, leads to premature menopause. It is often caused by hormonal issues such as adrenal, pituitary, or thyroid gland deficiencies. It can also be caused by genetic disorders such as Turner syndrome and fragile X syndrome. Certain types of autoimmune disorders (type 1 diabetes, systemic lupus erythematosus, Addison’s disease) are associated with premature ovarian failure. Radiation and chemotherapy cancer treatments can also cause early menopause.
- Hormonal Imbalances. Imbalances with reproductive hormones such as FSH, LH, estrogen, and progesterone can interfere with ovulation.
Blocked Fallopian Tubes
A blocked fallopian tube can prevent sperm from reaching and fertilizing the egg. Blockage in the fallopian tubes can also prevent a fertilized egg from traveling to the uterus for implantation. Conditions that can block or damage fallopian tubes include:
- Pelvic Inflammatory Disease (PID). PID refers to infection in the pelvic are and reproductive tract, including the fallopian tubes. PID is a complication of bacterial infection. The most common causes are sexually transmitted diseases, especially chlamydia and gonorrhea. In addition to infertility, PID can increase the risk for ectopic pregnancy, where the embryo implants in the fallopian tube or another location outside of the uterus.
- Endometriosis. Endometriosis is a condition in which cells that line the uterus grow in areas outside of the uterus, such as the ovaries. Endometriosis rarely causes a complete inability to conceive, but it can reduce fertility both directly and indirectly. Implants in the fallopian tubes may block the egg’s passage, while implants that occur in the ovaries can prevent the release of the egg. Severe endometriosis can form bands of scar tissue (adhesions) between the uterus, ovaries, and fallopian tubes that prevent egg transfer. [For more information, see In-Depth Report #74: Endometriosis.]
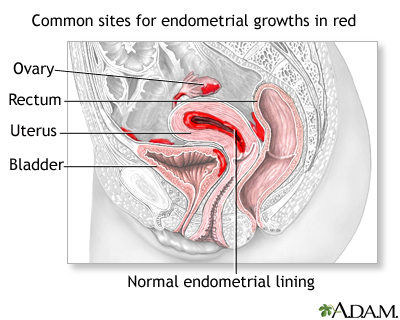
- Uterine or Abnormal Scarring. Adhesions can be caused by conditions besides endometriosis. Adhesions that form after abdominal or pelvic surgery or infection can restrict the movement of ovaries and fallopian tubes and may cause infertility. Asherman syndrome, for example, is scarring in the uterus that may be caused by surgery, repeated injury, or unknown factors.
- Uterine Fibroids. Uterine fibroids may contribute to infertility by blocking the fallopian tube, or by distorting the shape of the uterine cavity. Fibroids may also impair blood flow to the uterine lining. [For more information, see In-Depth Report #73: Uterine fibroids.]
Other Causes of Female Infertility
Other possible causes of female infertility include:
- Elevated Prolactin Levels. Prolactin is a hormone produced in the pituitary gland that stimulates breast development and milk production in association with pregnancy. High levels of prolactin (hyperprolactinemia) reduce gonadotropin hormones and inhibit ovulation. Hyperprolactinemia in women who are not pregnant or nursing can be caused by an underactive thyroid gland or pituitary adenoma. (Pituitary adenomas are benign tumors that secrete prolactin.) Some drugs, including oral contraceptives and some antipsychotic drugs, can also elevate levels of prolactin.
- Congenital Structural Abnormalities. Congenital reproductive tract abnormalities may cause infertility. These malformations typically affect the uterus or vagina. Daughters born to women who took the drug diethylstilbestrol (DES) during pregnancy are at increased risk of having uterine or fallopian tube structural abnormalities associated with infertility. Some women who are born with uterine or other reproductive tract malformations are still able to have successful pregnancies. Surgery can correct some of these problems.
- Cervical Mucus. Low amounts of cervical mucus or poor quality cervical mucus can contribute to infertility. Cervical mucus problems may be related to hormonal imbalances, prior surgeries, and certain medications.
- Egg Quality. As women age, the number and quality of their eggs diminish. Younger women can also have problems with egg quality, usually because of medical conditions or treatments that impair the ovaries. Radiation and chemotherapy cancer treatments, for example, can damage ovaries and affect egg development.
Risk Factors
In the U.S., about 10% of women ages 15 - 44, or about 6.1 million women, have problems getting pregnant or carrying a baby to term.
Age
Fertility declines as a woman ages. Fertility begins to decline when a woman reaches her mid-30s, and rapidly declines after her late 30s. As a woman ages, her ovaries produce fewer eggs. In addition, the quality of the eggs is poorer than those of younger women. Older women have a higher risk for eggs with chromosomal abnormalities, which increase the risk for miscarriage and birth defects. Older women are also more likely to have health problems that may interfere with fertility.
Weight
Although most of a woman's estrogen is manufactured in her ovaries, 30% is produced by fat cells, which transform male hormones produced by the adrenal glands into estrogen. Because a normal hormonal balance is essential for the process of conception, extreme weight levels (either high or low) can contribute to infertility.
Being Overweight. Being overweight or obese (fat levels that are 10 - 15% above normal) can contribute to infertility in various ways. Obesity is also associated with polycystic ovarian syndrome (PCOS), an endocrinologic disorder that can cause infertility.
Being Underweight. Body fat levels 10 - 15% below normal can completely shut down the reproductive process. Women at risk include:
- Women with eating disorders, such as anorexia or bulimia nervosa.
- Women on very low-calorie or restrictive diets are at risk, especially if their periods are irregular.
- Strict vegetarians might have difficulties if they lack important nutrients, such as vitamin B12, zinc, iron, and folic acid.
- Marathon runners, dancers, and others who exercise very intensely.
Smoking
Cigarette smoking can harm a woman’s ovaries and contribute to a decrease in eggs. Studies show that women who smoke are more likely to reach menopause earlier than women who do not smoke.
Alcohol and Caffeine Use
Alcohol and caffeine use may contribute to infertility.
Environmental Factors
Exposure to environmental hazards (such as herbicides, pesticides, and industrial solvents) may affect fertility. Estrogen-like chemicals that interfere with normal hormones are of particular concern for infertility in men and for effects on offspring of women. Phthalates, chemicals used to soften plastics, are under particular scrutiny because they may disrupt hormones.
Stress and Fertility
Neurotransmitters (chemical messengers in the brain) act in the hypothalamus gland, which controls both reproductive and stress hormones. It is not clear if stress has any significant effect on fertility or fertility treatments. Several large studies indicate it does not.
Diagnosis
In any fertility work-up, both male and female partners are tested if pregnancy fails to occur after a year of regular unprotected sexual intercourse. Fertility testing is particularly important if a woman is over 35 years old or if either partner has known risk factors for infertility. An analysis of the man's semen should be performed before the female partner undergoes any invasive testing.
Medical History and Physical Examination
The first step in any infertility work up is a complete medical history and physical examination. The doctor will ask about the patient's history of sexual activity, especially frequency and timing of intercourse. Menstrual history, lifestyle issues (smoking, drug and alcohol use, and caffeine consumption), any medications being taken, and a profile of the patient's general medical and emotional health can help the doctor decide on appropriate tests.
Easy Preliminary Steps
Before beginning an expensive fertility work-up, you can try the following steps. They are are free or low-cost and can be helpful:
- Monitor basal body temperature. This is accurate in determining if ovulation is actually taking place.
- Test the consistency of your cervical mucus. Collect some mucus between two fingers and stretch it apart. If you are near the time of ovulation, the mucus will stretch more than 1 inch before it breaks. As an alternative, at-home kits can test saliva as substitute for checking cervical mucus.
- Use an over-the-counter urine test to detect luteinizing hormone (LH) surges. This helps determine the day of ovulation. Tests are also available to measure levels of follicle-stimulating hormone (FSH). However, these at-home tests may not be as accurate as those performed in a doctor’s office.
Laboratory Tests
Several laboratory tests may be used to detect the cause of infertility and monitor treatments:
Hormonal Levels. Blood and urine tests are taken to evaluate hormone levels. Hormonal tests for ovarian reserve (the number of follicles and quality of the eggs) are especially important for older women.
Examples of possible results include:
- High follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels and low estrogen levels suggest premature ovarian failure.
- High LH and low FSH may suggest polycystic ovary syndrome or luteal phase defect.
- High FSH and high estrogen levels on the third day of the cycle predict poor success rates in older women trying fertility treatments.
- LH surges indicate ovulation.
- Blood tests for prolactin levels and thyroid function are also measured. These are hormones that may indirectly affect fertility.
Clomiphene Challenge Test. Clomiphene citrate (Clomid, Serophene, generic), a standard fertility drug, may be used to test for ovarian reserve. With this test, the doctor measures FSH on day 3 of the cycle. The woman takes clomiphene orally on days 5 and 9 of the cycle. The doctor measures FSH on the tenth day. High levels of FSH either on day 3 or day 10 indicate a poor chance for a successful outcome.
Tissue Samples. To rule out luteal phase defect, premature ovarian failure, or absence of ovulation, the doctor may take tissue samples of the uterus 1 - 2 days before a period to determine if the corpus luteum is adequately producing progesterone. Samples taken from the cervix may be cultured to rule out infection.
Tests for Autoimmune Disease. Tests for autoimmune disease, such as hypothyroidism and type 1 diabetes, should be considered in women with recent ovarian failure that is not caused by genetic abnormalities.
Imaging Tests and Diagnostic Procedures
If an initial fertility work-up does not reveal abnormalities, more extensive tests may help reveal abnormal tubal or uterine findings. The four major approaches for examining the uterus and fallopian tubes are:
- Ultrasound (particularly a variation called saline-infusion sonohysterography)
- Hysterosalpingography
- Hysteroscopy
- Laparoscopy
Combinations of these imaging procedures may be used to confirm diagnoses.
Ultrasound and Sonohysterography. Ultrasound is the standard imaging technique for evaluating the uterus and ovaries. It is also used for detecting fibroids, ovarian cysts and tumors, and obstructions in the urinary tract. It uses sound waves to produce an image of the organs and causes very little discomfort.
Transvaginal sonohysterography uses ultrasound along with saline infused into the uterus, which enhances the visualization of the uterus. This technique is proving to be more accurate than standard ultrasound in identifying potential problems. It is currently the gold standard for diagnosing polycystic ovaries.
Hysteroscopy. Hysteroscopy is a procedure that may be used to detect the presence of endometriosis, fibroids, polyps, pelvic scar tissue, and blockage at the ends of the fallopian tubes. Some of these conditions can be corrected during the procedure by cutting away any scar tissue that may be binding organs together or by destroying endometrial implants.
Hysteroscopy may be done in a doctor’s office or in an operating room, depending on the type of anesthesia used. The procedure uses a long flexible or rigid tube called a hysteroscope, which is inserted into the vagina and advanced through the cervix to reach the uterus. A fiber-optic light source and a tiny camera in the tube allow the doctor to view the cavity. The uterus is filled with saline or carbon dioxide to inflate the cavity and provide better viewing. This can cause cramping.
There are small risks of bleeding, infection, and reactions to anesthesia. Many patients experience temporary discomfort in the shoulders after the operation due to residual carbon dioxide that puts pressure on the diaphragm.
Hysterosalpingography. Hysterosalpingography is performed to discover possible blockage in the fallopian tubes and abnormalities in the uterus:
- The doctor inserts a tube into the cervix through which a special dye is injected. (The patient may experience some cramping and discomfort.)
- The dye passes into the uterus and up through the fallopian tubes.
- An x-ray is taken of the dye-filled uterus and tubes.
- If the dye is seen emerging from the end of the tube, no blockage is present. (In some cases, hysterosalpingography may even restore fertility by clearing away tiny tubal blockages.)
- If results show blockage or abnormalities, the test may need to be repeated. In case of blockage, hysterosalpingography may reveal a number of conditions, including endometrial polyps, fibroid tumors, or structural abnormalities of the uterus and tubes.
There is a small risk of pelvic infection, and antibiotics may be prescribed prior to the procedure.
Laparoscopy. Laparoscopy is a minimally invasive surgical procedure. It requires general anesthesia and is performed in an operating room. The surgeon makes a very small incision below the belly button and inserts an instrument called a laparoscope, which is similar to a hysteroscope. (The difference is that a laparoscope is inserted through the abdomen, while a hysteroscope is inserted through the vagina and cervix.) Through the laparoscope, the surgeon can view the uterus, fallopian tube, and ovaries. Laparoscopy is most helpful for identifying endometriosis or other adhesions that may affect fertility.
Treatment
Treatment for infertility should first address any underlying medical condition that may be contributing to fertility problems. Drugs, surgery, or both may be used to treat these conditions. Surgery may also be used to repair blockage in fallopian tubes.
Fertility Treatment Approaches
Several approaches are used to treat infertility:
- Lifestyle measures (such as maintaining a healthy weight, quitting smoking, refraining from excessive alcohol use, timing sexual activity with ovulation cycle)
- Drugs to induce ovulation, such as clomiphene and gonadotrophins
- Assisted reproductive technologies (ART) such as in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI)
Choosing a Fertility Clinic
Some doctors recommend that if a couple fails to conceive after 1 - 2 years of frequent unprotected sex, they should consult a fertility expert. Women who are 35 or older, however, may want to begin exploring their options if they do not become pregnant within 6 months to a year.
Choosing a good fertility clinic is important. Those offering assisted reproductive techniques are not always regulated by the government, and abuses have been reported, including lack of informed consent, unauthorized use of embryos, and failure to routinely screen donors for disease.
The clinic should always provide the following information:
- The live-birth rate (not just pregnancy success rate) for other couples with similar infertility problems. (Multiple births, such as twins or triplets, are counted as one live birth.)
- Such statistics should include high-risk women, such as those who are older or fail to produce eggs. (Some disreputable clinics give success percentages that exclude high-risk women from their total, thereby making the percentage of success much higher.)
Advanced fertility procedures and medications are extremely expensive and often not covered by insurance. Couples should be cautious about offers of rebates in the event of failure; the clinics offering them are often significantly more expensive than those that don't offer such gimmicks.
Special Considerations for Patients with Cancer
Women who are undergoing cancer treatments and who want to become pregnant should see a reproductive specialist to discuss their options. According to the American Society of Clinical Oncology's guidelines, the fertility preservation method with the best chance of success is embryo cryopreservation. This procedure involves harvesting a woman's eggs (oocytes), followed by in vitro fertilization and freezing of embryos for later use. Other treatments under investigation include egg preservation, collecting and freezing unfertilized eggs, removing and freezing a part of the ovary for later reimplantation, and using hormone therapy to protect the ovaries during chemotherapy. Women may be able to access these investigational approaches through enrolling in clinical trials.
Medications
Fertility drugs are often used alone as initial treatment to induce ovulation. If they fail as sole therapy, they may be used with assisted reproductive procedures, such as in vitro fertilization, to produce multiple eggs, a process called superovulation.
According to the American Society for Reproductive Medicine, fertility drugs can be divided into three main categories:
- Medications for Ovarian Stimulation. Clomiphene (Clomid, Serophene, generic); letrozole (Femara), follicle stimulating hormone (FSH) [Follistim, Gonal-F, Bravelle]; human menopausal gonadotrophin (hMG) [Humegon, Repronex, Menopur]; luteneizing hormone (LH) [Luveris]
- Medications for Oocyte Maturation. Human chorionic gonadotropin (hCG) [Profasi, APL, Pregnyl, Novarel, Ovidrel]
- Medications to Prevent Premature Ovulation. GnRh agonists [leuprolide (Lupron, generic), nafarelin (Synarel), and goserelin (Zoladex)]; Gn RH antagonists [ganirelix (Antagon), cetrorelix (Cetrotide)].
Clomiphene
Clomiphene citrate (Clomid, Serophene, generic) is usually the first fertility drug prescribed for women with infrequent periods and long menstrual cycles. Unlike more potent drugs used in superovulation, clomiphene is gentler and works by blocking estrogen, which tricks the pituitary into producing follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This boosts follicle growth and the release of the egg. Clomiphene can be taken by mouth, is relatively inexpensive, and the risk for multiple births (about 5%, mostly twins) is lower than with other drugs.
Women with the best chances for success with this drug are those who have the following conditions:
- Polycystic ovarian syndrome (PCOS)
- Ability to menstruate but irregular menstrual cycle
Women with poorer chances of success with this drug have the following conditions:
- Infertility but with normal ovulation
- Low estrogen levels
- Premature ovarian failure (early menopause)
One or two tablets are taken each day for 5 days, usually starting 2 - 5 days after the period starts. If successful, ovulation occurs about a week after the last pill has been taken. If ovulation does not occur, then a higher dose may be given for the next cycle. If this regimen is not successful, treatment may be prolonged or additional drugs may be added. Doctors usually do not recommend more than 6 cycles.
Clomiphene often reduces the amount and quality of cervical mucus and may cause thinning of the uterine lining. In such cases, other hormonal drugs may be given to restore thickness. Other side effects of clomiphene include ovarian cysts, hot flashes, nausea, headaches, weight gain, and fatigue. There is a 5% chance of having twins with this drug, and a slightly increased risk for miscarriage.
Gonadotropins
If clomiphene does not work or is not an appropriate choice, gonadotropin drugs are a second option. Gonadotropins include several different types of drugs that contain either a combination of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), or only FSH. Whereas clomiphene works indirectly by stimulating the pituitary gland to secrete FSH, (which prompts follicle production), gonadtropin hormones directly stimulate the ovaries to produce multiple follicles.
Gonadotropins are given by injection. (Your doctor may show you how to self-administer the injection.) Gonadotropins include:
- Human Menopausal Gonadtropins (hMG), also called menotropins
- Human Chorionic Gonadotropins (hCG)
- Follicle Stimulating Hormone (FSH)
- Gonadotropin-releasing hormone (GnRH) analogs, which include GnRH agonists and GnRH antagonists
Gonadotropin drugs are either natural compounds extracted from urine or synthetic compounds that are genetically engineered in a laboratory using recombinant DNA.
Human Menopausal Gonadotropin (hMG). HMG drugs, also called menotropins, contain a mixture of both FSH and LH. These drugs (Menopur, Repronex, Humegon) are all derived from the urine of postmenopausal women. HMG is administered as a series of injections 2 - 3 days after the period starts. Injections are usually given for 7 - 12 days, but the time may be extended if ovulation does not occur. In such cases, a shot of human chorionic gonadotropin (hCG) may trigger ovulation.
Human Chorionic Gonadotropin (hCG). Human chorionic gonadotropin (hCG) is similar to LH. It mimics the LH surge, which stimulates the follicle to release the egg. Natural hCG drugs, derived from the urine of pregnant women, include Pregnyl, Profasi, Novarel, and APL. Ovidrel is the only available genetically modified hCG drug. Ovidrel has fewer side effects at the injection site, and its quality can be better controlled than the natural drugs. It is generally used after hMG or FSH to stimulate the final maturation stages of the follicles. Ovulation, if it occurs, does so about 36 - 72 hours after administration.
Follicle Stimulating Hormone (FSH). Urofollitropin (Bravelle, Fertinex) is a purified form of FSH, derived from the urine of postmenopausal women. Follitropin drugs (Gonal-F, Follistim) are synthetic versions of FSH. These FSH drugs are sometimes given in combination with an hCG drug.
GnRH Analogs (Agonists or Antagonists). Gonadotropin-releasing hormone (GnRH) is a hormone produced in the hypothalamus part of the brain. GnRH stimulates the pituitary gland to produce LH and FSH. GnRH analogs are synthetic drugs that are classified as either agonists or antagonists. They are similar to natural GnRH but have very different actions. While natural GnRH stimulates LH and FSH, these drugs actually prevent the LH and FSH surge that occurs right before ovulation. This action helps prevent the premature release of the eggs before they can be harvested for assisted reproductive technologies.
- GnRH agonists include leuprolide (Lupron, generic), nafarelin (Synarel), and goserelin (Zoladex).
- GnRH antagonists include ganarelix (Antagon) and cetrorelix (Cetrotide). GnRH antagonists suppress FSH and LH more than GnRH agonists, and they may require fewer injections.
Complications of Superovulation
Multiple Births. Overproduction of follicles can lead to ovarian enlargement. This event increases the risk for multiple births. There is a 25% chance of multiple births (about 17% for twins and 8% for triplets and or more).
Ovarian Hyperstimulation Syndrome. The most serious complication with superovulation is ovarian hyperstimulation syndrome (OHS), which is associated with the enlarged ovary (although the precise cause is unknown). This can result in dangerous fluid and electrolyte imbalances and endanger the liver and kidney. OHS is also associated with a higher risk for blood clots. In rare cases, it can be fatal. Symptoms include abdominal bloating, nausea, vomiting, and shortness of breath.
Bleeding and Rupture of Ovarian Cysts. Overproduction of follicles, if unchecked, may result in bleeding and rupture of ovarian cysts.
Cancer Concerns. There has been concern that clomiphene and gonadotropins may increase the risks for ovarian and breast cancer. Most evidence to date does not indicate that ovulation-stimulating drugs increase the risks for these types of cancers. Some studies suggest that clomiphene, which is chemically related to the breast cancer drug tamoxifen, may actually decrease the risk for breast cancer.
Other Drugs Used or Under Investigation
Letrozole and Aromatase Inhibitors. Aromatase inhibitors block aromatase, an enzyme that is largely responsible for producing estrogen in body tissues outside of the ovaries. These drugs include anastrozole (Arimidex) and letrozole (Femara). These drugs are used for treating breast cancer and are being investigated for stimulating ovulation in infertile women. Although letrozole is not approved for treatment of infertility, it has become widely used for this purpose in recent years.
Progesterone. Progesterone is a hormone that is produced by the body during the menstrual cycle. Progesterone drugs are sometimes given to women who have experienced frequent miscarriages (a possible sign of progesterone deficiency). A progesterone drug may also be given after egg retrieval during an in vitro fertilization (IVF) cycle to help thicken the uterine lining (endometrium) so it can better hold the egg following implantation.
Tamoxifen. Tamoxifen (Nolvadex, generic) is a drug known as a selective estrogen-receptor modulator (SERM). It is used to treat or prevent breast cancer in certain women. It is also being studied in fertility treatments to induce ovulation. Tamoxifen works in a similar to clomiphene but may pose more health hazards, including a risk for blood clots and uterine cancer.
Glucocorticoids. Glucocorticoids are steroid hormones that are sometimes used in combination with IVF and intracytoplasmic sperm injection (ICSI) to help make the lining of the uterus more responsive to egg implantation. However, recent reviews caution that glucocorticoids do not help improve pregnancy success rates and should not be used routinely with assisted reproductive technologies.
Assisted Reproductive Technologies
Assisted reproductive technologies (ART) are medical techniques that help couples conceive. These procedures involve either:
- A couple’s own eggs or sperm
- Donor eggs, sperm, or embryos
Fertilization may occur either in the laboratory or in the uterus. In the U.S., the number of live birth deliveries from ART has dramatically increased in the last decade. About 45,000 live births (deliveries of one or more infants) occur in the U.S. each year using assisted reproductive technologies.
Technically, the term ART refers only to fertility treatments, such as in vitro fertilization (IVF) and its variants, which handle both egg and sperm.
Intrauterine Insemination (IUI)
Artificial insemination (AI) involves placing the sperm directly in the cervix (called intracervical insemination) or into the uterus (called intrauterine insemination, or IUI).
IUI is the standard AI procedure. It involves placing washed sperm into the woman’s uterine cavity through a long, thin catheter. The procedure is performed when a woman is ovulating and is used for patients whose tubes are not blocked from endometriosis or scarring.
- A woman will first be given three cycles of clomiphene pills to stimulate egg production, at the same time having sexual contact with her partner timed around ovulation.
- If she fails to conceive, three additional cycles of clomiphene are used, combined with IUI.
- If the clomiphene-IUI combination does not work, the woman receives three cycles of IUI combined with injections of a gonadotropin-stimulating hormone drug.
- If this method fails, the woman may be a candidate for in vitro fertilization (IVF). Some insurance plans only cover IUI and do not pay for IVF.
IUI is the least complex and least expensive of fertility procedures and is often tried first in uncomplicated cases of infertility. However, recent studies indicate that IUI combined with fertility injections poses a greater risk for multiple births and has a lower rate of pregnancy success than IVF.
For these reasons, some doctors now suggest that women skip the gonadotropin-stimulating injection step, and that couples who fail to conceive after three cycles of IUI combined with clomiphene pills proceed directly to IVF.
In Vitro Fertilization (IVF)
Most assisted reproductive technologies procedures use in vitro fertilization (IVF). An in vitro procedure is one that is performed in the laboratory. Advances in these procedures have dramatically increased the rate of live births. IVF can be performed with a woman’s own eggs and sperm, or with donor eggs and sperm.
In the past, IVF was used mainly to treat women with damaged fallopian tubes. It is now used as a fertility treatment for cases when the woman has endometriosis, the man has fertility problems, or the cause of a couple’s infertility is unexplained.
A standard IVF cycle is divided into the following steps:
- Ovarian Stimulation. Ovarian-stimulating drugs, such as clomiphene, are used to prompt the ovaries to produce multiple eggs. About 8 - 14 days later, another type of drug [usually human chorionic gonadotropin (hCG)] is given to foster egg maturation.
- Egg Retrieval. About 34 - 36 hours after the hCG injection, the eggs are retrieved before ovulation begins. A cycle may be canceled at this stage if not enough follicles are produced or if there is a risk of ovarian hyperstimulation syndrome (see Medications section).To retrieve the eggs, the doctor inserts an ultrasound-guided probe into the vagina. A needle is then used to drain the liquid from the follicles, and several eggs are retrieved.
- Fertilization and Embryo Culture. The doctor will examine the eggs to evaluate their quality and maturity. Selected eggs are placed in a culture in the laboratory and transferred to an incubator. They are then inseminated with sperm, either by placing sperm together with the egg or injecting a single sperm into the egg (see ICSI section below).
- Embryo Transfer and Cryopreservation. One or more embryos are implanted in the woman’s uterus 1 - 6 days after egg retrieval. The doctor will discuss with the patient the appropriate number of embryos to be implanted. Excess embryos may be frozen and saved for future use. (The live birth rate is usually lower with cryopreserved embryos.) It takes about 2 weeks to determine if pregnancy has been achieved.
Embryo Transfer Guidelines. The American Society for Reproductive Medicine (ASRM) and the Society for Assisted Reproductive Technologies (SART) have joint guidelines on the number of embryos that should be transferred during IVF procedures:
- For women under the age of 35, a single embryo
- For women between ages 35 - 37, no more than two embryos
- For women between ages 38 - 40, no more than three embryos
- For women ages 41 - 42, no more than five embryos
- For women older than age 42, there are insufficient data to recommend a limit on the number of embryos
These embryo numbers are recommended for women with favorable prognoses. For patients who have failed to become pregnant after at least two IVF cycles, or who have a less favorable prognosis, the doctor may consider adding one additional embryo. The guidelines apply to both fresh and frozen embryos.
Other IVF Procedures. About 1 - 2% of IVF procedures use adaptations called gamete intrafallopian transfer (GIFT) and zygote intrafallopian transfer (ZIFT), which transfers the gametes (egg and sperm) into a women’s fallopian tube rather than her uterus. In GIFT, the egg is harvested as with IVF and mixed with sperm, and is then injected into the woman’s fallopian tube where fertilization occurs. In ZIFT, the egg is fertilized with sperm in the laboratory before being transferred to the fallopian tube. For GIFT and ZIFT a woman must have at least one functioning fallopian tube.
Success Rates. Not all IVF cycles result in pregnancy, and not all IVF-achieved pregnancies result in live births. When a woman’s own eggs are used, results are better with fresh embryos than frozen embryos. According to the most recent statistics from the U.S. Centers for Disease Control (CDC), about 31% of ART cycles (mostly IVF) with fresh embryos resulted in a live birth of one or more babies. Success rates provided by fertility clinics are not always a reliable indicator as they depend on many variables, especially the age of the woman.
Data indicate that the chances of IVF resulting in live birth are about:
- 40% for women younger than age 35
- 30% for women ages 35 - 37
- 20% for women ages 38 - 40
- 10% for women ages 41 - 42
- 5% for women ages 43 - 44
Some women try acupuncture during an IVF cycle to increase their chances for pregnancy success. While acupuncture is not harmful, there is no conclusive evidence that it boosts success rates.
Complications. Data have been conflicting on whether IVF increases the risk for genetic abnormalities and birth defects. In general, the overall risks for birth defects appear to be small. Studies indicate that most children conceived through IVF are healthy and have normal cognitive development and school performance.
The main risk of IVF is the consequences of multiple pregnancies. Multiple pregnancies increase the risks for a mother and her babies. In particular, there is increased risk for premature delivery and low birth weight. These factors can cause heart and lung problems and developmental disabilities in children.
Intracytoplasmic Sperm Injection (ICSI)
Intracytoplasmic sperm injection (ICSI) is an assisted reproductive technology used for couples when male infertility is the main problem. It involves injecting a single sperm into an egg obtained from in vitro fertilization (IVF). The procedure is very simple:
- A tiny glass tube (called a holding pipet) stabilizes the egg.
- A second glass tube (called the injection pipet) is used to penetrate the egg's membrane and deposit a single sperm into the egg.
- The egg is released into a drop of cultured medium.
- If fertilized, the egg is allowed to develop for 1 - 2 days, then it is either frozen or implanted.
The greatest concern with this procedure has been whether it increases the risk for birth defects. Many, but not all, studies have reported no higher risks of birth defects in children born using ICSI procedures. However, if the father’s infertility was due to genetic issues, this genetic defect may be passed on to male children conceived through ICSI.
Another concern has been whether the ICSI procedure is being overused. ICSI use has increased 5-fold over the past decade and is now used in 65% of ART procedures, even though the proportion of men receiving treatment for male infertility has remained the same. Some doctors recommend ICSI for women who have failed prior IVF attempts or who have few or poor-quality eggs, even if their male partners have normal semen measurements. According to the Society for Assisted Reproductive Technology, there is little evidence that ICSI helps improve pregnancy success for couples who do not have a problem with male factor infertility.
Lifestyle Changes
Although there are no dietary or nutritional cures for infertility, a healthy lifestyle is important. Some ovulatory problems may be reversible by changing behavioral patterns. Some tips include:
- Maintain a healthy weight. Women who are either over- or underweight are at risk for fertility failure, including a lower chance for achieving success with fertility procedures.
- Stop smoking. Smoking may increase the risk for infertility in both men and women. Everyone should quit.
- Avoid excessive exercise if it causes menstrual irregularity. However, moderate and regular exercise is essential for good health.
- Avoid or limit caffeine and alcohol.
- Avoid any unnecessary medications.
Planning Sexual Activity and Monitoring Basal Body Temperature
Both male and female hormone levels fluctuate according to the time of day, and they vary from day to day, month to month, and seasonally. Some timing tips might be helpful.
Monitoring Basal Body Temperature. To determine the most likely time of ovulation and therefore the time of fertility, a woman should take her body temperature, called her basal body temperature. This is the body's temperature as it rises and falls in accord with hormonal fluctuations.
- Each morning before rising, the woman takes her temperature with a specialized basal body thermometer and marks the result on a graph-paper chart.
- The woman also notes the days of menstruation and sexual activity.
- The so-called "fertile window" is 6 days long, starts 5 days before ovulation, and ends the day of ovulation.
- The chances for fertility are considered to be highest between days 10 and 17 in the menstrual cycle (with day 1 being the first day of the period, and ovulation occurring about 2 weeks later). However, cycles vary from woman to woman.
- Immediately after ovulation the body temperature increases sharply in about 80% of cases. (Some women can be ovulating normally yet not show this temperature pattern.)
By studying the temperature patterns after a few months, couples can begin to anticipate ovulation and plan their sexual activity accordingly. Couples should try to avoid becoming fixated on the chart, however, in scheduling their sexual activity.
Frequency of Intercourse. It is not clear how often a couple should have intercourse in order to conceive. Some doctors think that having sex more than 2 days a week adds no benefits. In addition, frequent sexual activity lowers sperm count per ejaculation. Some studies have indicated, however, that having intercourse every day, or even several times a day, before and during ovulation, improves pregnancy rates. Although sperm count per ejaculation is low, a constantly replenished semen supply is more likely to result in a fertilized egg.
Dealing with Stress
The fertility treatment process presents a roller coaster of emotions. There are almost no sure ways to predict which couples will eventually conceive. Some couples with multiple problems will overcome great odds, while other, seemingly fertile, couples fail to conceive. Many of the new treatments are remarkable, but a live birth is never guaranteed. The emotional burden on the couple is considerable, and some planning is helpful.
- Decide in advance how many and what kind of procedures will be emotionally and financially acceptable and attempt to determine a final limit. Fertility treatments are expensive. A successful pregnancy often depends on repeated attempts.
- Prepare for multiple births as a possible outcome for successful pregnancy (especially if assisted reproductive technologies are used). A pregnancy that results in a multiple birth introduces new complexities and emotional problems
- Determine alternatives (adoption, donor sperm or egg, or having no children) as early as possible in the fertility process. This can reduce anxiety during treatments and feelings of disappointment in case conception does not occur.
On a reassuring note, a large study of infertile women indicated that stress levels do not affect the outcome of fertility treatments. The study found no difference in stress levels between women who became pregnant and those who did not. Women who are feeling stressed by problems with fertility or the challenges of the fertility treatment process should not feel additionally concerned that their emotional state may affect their chances of becoming pregnant.
Resources
- www.asrm.org -- American Society for Reproductive Medicine
- www.sart.org -- Society for Assisted Reproductive Technology
- www.theafa.org -- American Fertility Association
- www.acog.org -- American College of Obstetricians and Gynecologists
- www.cdc.gov/reproductivehealth/index.htm -- Centers for Disease Control: Assisted Reproductive Technology Reports
References
Boivin J, Griffiths E, Venetis CA. Emotional distress in infertile women and failure of assisted reproductive technologies: meta-analysis of prospective psychosocial studies. BMJ. 2011 Feb 23;342:d223. doi: 10.1136/bmj.d223.
Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012 May 10;366(19):1803-13. Epub 2012 May 5..
El-Toukhy T, Sunkara SK, Khairy M, Dyer R, Khalaf Y, Coomarasamy A. A systematic review and meta-analysis of acupuncture in in vitro fertilisation. BJOG. 2008 Sep;115(10):1203-13. Epub 2008 Jul 23.
ESHRE Capri Workshop Group. Intrauterine insemination. Hum Reprod Update. 2009 May-Jun;15(3):265-77. Epub 2009 Feb 23.
Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011 Oct 6;365(14):1304-14.
Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol. 2010 Nov;116(5):1171-83.
Jain T, Gupta RS. Trends in the use of intracytoplasmic sperm injection in the United States. N Engl J Med. 2007 Jul 19;357(3):251-7.
Jensen A, Sharif H, Frederiksen K, Kjaer SK. Use of fertility drugs and risk of ovarian cancer: Danish Population Based Cohort Study. BMJ. 2009 Feb 5;338:b249. doi: 10.1136/bmj.b249.
Jensen JR, Morbeck DE, Coddington CC 3rd. Fertility preservation. Mayo Clin Proc. 2011 Jan;86(1):45-9.
Jensen A, Sharif H, Svare EI, Frederiksen K, Kjaer SK. Risk of breast cancer after exposure to fertility drugs: results from a large Danish cohort study. Cancer Epidemiol Biomarkers Prev. 2007 Jul;16(7):1400-7. Epub 2007 Jun 21.
Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006 Jun 20;24(18):2917-31.
Luke B, Brown MB, Wantman E, Lederman A, Gibbons W, Schattman GL, et al. Cumulative birth rates with linked assisted reproductive technology cycles. N Engl J Med. 2012 Jun 28;366(26):2483-91.
Mains L, Zimmerman M, Blaine J, Stegmann B, Sparks A, Ansley T, et al. Achievement test performance in children conceived by IVF. Hum Reprod. 2010 Oct;25(10):2605-11. Epub 2010 Aug 17.
Manheimer E, Zhang G, Udoff L, Haramati A, Langenberg P, Berman BM, Bouter LM. Effects of acupuncture on rates of pregnancy and live birth among women undergoing in vitro fertilisation: systematic review and meta-analysis. BMJ. 2008 Mar 8;336(7643):545-9. Epub 2008 Feb 7.
Practice Committee of the American Society for Reproductive Medicine; Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009 Nov;92(5):1518-9. Epub 2009 Oct 17.
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009 Nov;92(5):1520-4. Epub 2009 Oct 14.
|
Review Date:
12/19/2012 Reviewed By: Harvey Simon, MD, Editor-in-Chief, Associate Professor of Medicine, Harvard Medical School; Physician, Massachusetts General Hospital. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M., Inc. |